Potential Energy Diagram Covalent Bond
Why does the potential energy get lower as atoms get closer? Potential energy graph hydrogen molecule distance internuclear chemistry where atoms point has zero Potential energy diagrams for formation of bonds
Potential Energy Diagrams For Formation Of Bonds | Mini Physics - Free Physics Notes
Energy potential bond diagram covalent formation waals der van bonds diagrams graph binding physics Potential energy diagrams for formation of bonds Covalent bond
8.9: covalent bond properties: order, length, and energy
Potential energy diagrams for formation of bondsCovalent formation waals bonds physics binding diagrams miniphysics Bond covalent energy potential bonding theory two lewis diagram atoms formation adichemistry between difference model when generalCovalent bonding teaching resources – the science teacher.
Chapter 4.1: ionic bondingCovalent bond Inorganic chemistryBond energy chemical bonding length formation break required ppt powerpoint presentation.

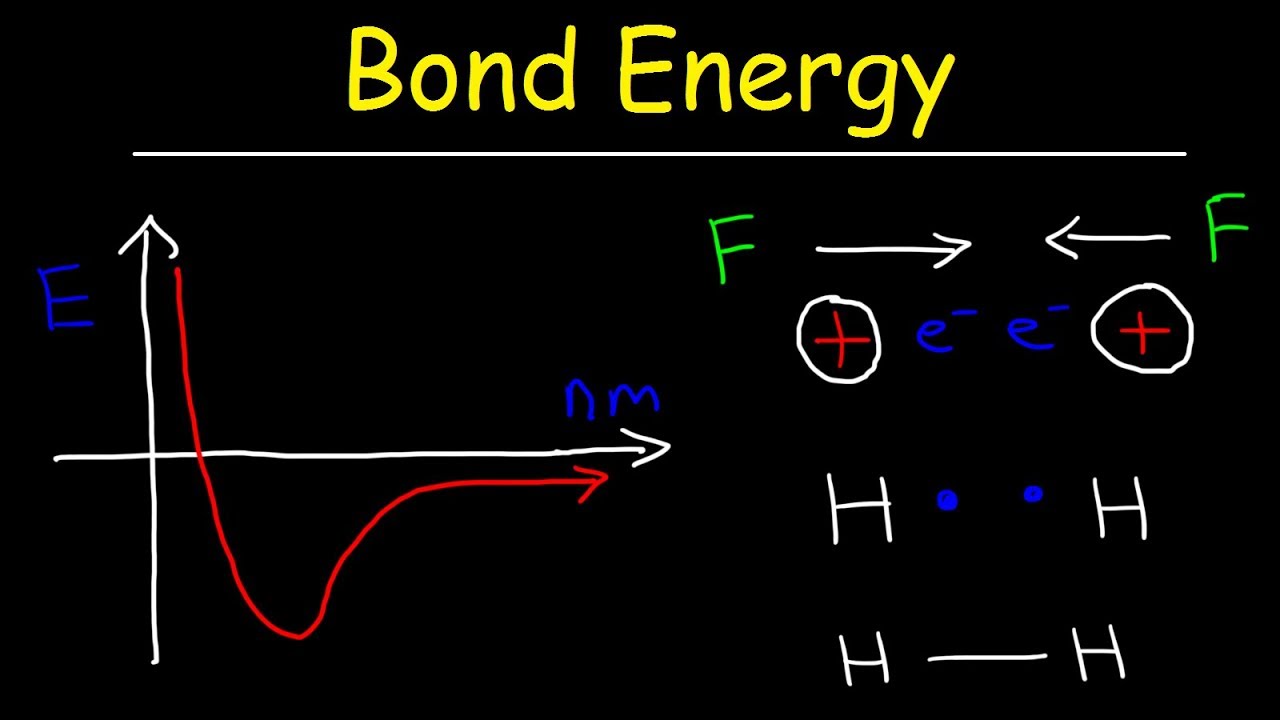
Bond energy & bond length, forces of attraction & repulsion
Energy potential bond atoms covalent formation bonds two hydrogen distance chemistry graph separation changes electron bonding function shows water theirEnergy potential bond covalent formation waals bonds binding diagrams miniphysics explanation Potential energy curves (interaction potential v vs ar-br2 distance r)...Ch 2: bonding.
Covalent bonds bond energy bonding ionic two chemistry graph potential chem distance internuclear atoms vs hydrogen energies line single labeledChemistry energy potential bond chemical two covalent hydrogen bonding atoms electron versus between diagram valence theory lewis structures water distance Energy and covalent bond formationBr2 interaction.
Dissociate atoms principles gillis oxtoby
Strengths of ionic and covalent bondsEnergy ion versus ionic bonding covalent chemical chemistry interactions bond distance when lattice system minimum basic potential interaction diagram internuclear Potential energy distance graph between atoms why does closer nucleus lower plot variation get bond nuclei show read two kineticBond energy length chemistry forces attraction repulsion.
Covalent energy bond h2 virial 2008 analysis interaction formation graph csbsju december eduBond covalent potential energy bonding concept general formation diagram ppt powerpoint presentation Bond formation energy potential bonding two orbitals curve atoms 1s interaction when ucalgary courses chem ch02 ca molecular electron sigmaBond energy length potential covalent energies atoms lengths order two breaking molecule when why distance bonds curve formation between chemistry.

Covalent bonding teaching resources – the science teacher
Why does the potential energy get lower as atoms get closer?

Strengths of Ionic and Covalent Bonds | Chemistry

PPT - Bonding: General Concept PowerPoint Presentation, free download - ID:4507469

COVALENT BOND | LEWIS BONDING THEORY | DOT MODEL | ADICHEMISTRY

Potential Energy Diagrams For Formation Of Bonds | Mini Physics - Free Physics Notes

Energy and Covalent Bond Formation | Chemistry for Non-Majors

atoms - Hydrogen molecule potential energy graph - Chemistry Stack Exchange

8.9: Covalent Bond Properties: Order, Length, and Energy - Chemistry LibreTexts